Articles from JCR Pharmaceuticals Co., Ltd.

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, today announced the presentation of four datasets demonstrating the potential benefits of its investigational therapies for lysosomal storage disorders (LSDs) at the 22nd Annual WORLDSymposiumTM 2026. Researchers are presenting new data from two of its programs that apply the J-Brain Cargo® platform, a proprietary technology developed by JCR to deliver medicines across the blood-brain barrier (BBB), through four poster presentations this week.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 4, 2026

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, announced today that it will present data at the 22nd Annual WORLDSymposium™ 2026, held February 2-6, 2026, in San Diego, Calif. The poster presentations will demonstrate the potential benefits of the investigational therapies in JCR’s development pipeline and of J-Brain Cargo®, JCR’s proprietary technology that delivers medicine across the blood-brain barrier (BBB) for the treatment of lysosomal storage disorders and other neurodegenerative diseases.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · January 27, 2026

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, announced today that it will present at the 44th Annual J.P. Morgan Healthcare Conference taking place January 12–15, 2026.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · January 8, 2026

Italfarmaco S.p.A. and JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), today announced an exclusive licensing agreement for the development and commercialisation of givinostat in Japan.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · December 24, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, today presented non-clinical data demonstrating the ability of its proprietary JUST-AAV capsid engineering platform to achieve efficient delivery of adeno-associated virus (AAV) gene therapy to the central nervous system (CNS) and muscle while reducing liver exposure. The data, presented at the European Society of Gene and Cell Therapy (ESGCT) 32nd Annual Congress, in Seville, Spain, showcase the platform’s potential to overcome longstanding challenges in treating CNS and muscle disorders.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · October 7, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, today announced an exclusive licensing agreement with Menagen Pharmaceutical Industries LLC (headquartered in Oman, CEO: Saif Al Hasani, “Menagen”) to seek local marketing authorizations and to commercialize Agalsidase Beta BS I.V. Infusion [JCR] (the “product”) upon approval across nine MENAT markets. The product is a recombinant enzyme replacement therapy (ERT) for Fabry disease currently marketed in Japan.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · October 1, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, announced today that it will present non-clinical data from its novel JUST-AAV gene therapy platform technology in an oral session at the European Society of Gene and Cell Therapy (ESGCT) 32nd Annual Congress, being held October 7-10, 2025, in Seville, Spain.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 30, 2025

MEDIPAL HOLDINGS CORPORATION (TSE 7459, MEDIPAL) and JCR Pharmaceuticals Co., Ltd. (TSE 4552, JCR) today announced that the Ministry of Health, Labour and Welfare of Japan has granted orphan drug designation to JR-446, an investigational drug for the treatment of mucopolysaccharidosis type IIIB (MPS IIIB or Sanfilippo syndrome type B).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 29, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, has achieved a key research milestone in collaboration with Alexion, AstraZeneca Rare Disease (“Alexion”), focused on applying JCR’s proprietary J-Brain Cargo® technology to develop a therapeutic protein candidate for a neurodegenerative disease. The achievement triggers a milestone payment from Alexion to JCR.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 19, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, today announced that it will celebrate its 50th anniversary on September 13, 2025, marking five decades of advancing science and redefining possibilities for patients living with complex healthcare challenges. The company expresses its deep gratitude to patients, families, partners, and stakeholders worldwide who have been part of its journey.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 12, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, presented new data from its pipeline of brain-penetrant enzyme replacement therapies at the 15th International Congress of Inborn Errors of Metabolism (ICIEM) in Kyoto, Japan, held September 2-6, 2025. Key findings included five-year clinical data demonstrating sustained neurocognitive and somatic benefits of pabinafusp alfa (JR-141) in patients with mucopolysaccharidosis type II (MPS II, or Hunter syndrome), suggesting improvements in patients treated prior to significant neuronopathy and stabilization in those with advanced disease. Additional posters highlighted five years of safety data of pabinafusp alfa in MPS II, preclinical cardiovascular effects of pabinafusp alfa in MPS II, and nonclinical efficacy of JR-446, an investigational therapy for mucopolysaccharidosis type IIIB (MPS IIIB, also known as Sanfilippo syndrome type B).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 8, 2025

MEDIPAL HOLDINGS CORPORATION (TSE 7459, MEDIPAL) and JCR Pharmaceuticals Co., Ltd. (TSE 4552, JCR) announced that the two companies have signed an exclusive global licensing deal and a co-development and commercialization partnership in Japan for JR-479, an investigational therapy for GM2 gangliosidosis, an ultra-rare lysosomal storage disorder.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 2, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, announced today that it will present data at the 15th International Congress of Inborn Errors of Metabolism (ICIEM) 2025, held September 2-6, 2025, in Kyoto, Japan. The presentations will demonstrate the potential benefits of the investigational therapies in JCR’s development pipeline and of J-Brain Cargo®, JCR’s proprietary technology that delivers medicine across the blood-brain barrier (BBB) for the treatment of lysosomal storage disorders and neurodegenerative disorders.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · August 28, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, has been selected for the Ministry of Economy, Trade and Industry’s “Regenerative CDMO Subsidy” as of July 15, 2025. The subsidy will support facility upgrades and equipment installation to expand biomanufacturing capacity for regenerative, cell, and gene therapies.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · July 16, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; JCR), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, today announced a joint collaboration, option and license agreement with Acumen Pharmaceuticals to develop a novel therapeutic candidate for the treatment of Alzheimer’s disease (AD), enabled by JCR’s proprietary blood-brain barrier (BBB)-penetrating technology platform, J-Brain Cargo®.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · July 15, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) today announced that it has entered into a license agreement with Alexion, AstraZeneca Rare Disease (“Alexion”), for JCR’s new, proprietary JUST-AAV capsids to develop genomic medicines.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · July 8, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; JCR) announced that it achieved the enrollment of the target number of participants in the global Phase III clinical trial of JR-141 (INN: pabinafusp alfa), which is in development for the treatment of mucopolysaccharidosis type II (MPS II, also known as Hunter syndrome). The Phase III clinical trial is ongoing in the United States, Latin America, and Europe. (JR-141-GS31)
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · July 2, 2025
MEDIPAL HOLDINGS CORPORATION (TSE 7459, MEDIPAL) and JCR Pharmaceuticals Co., Ltd. (TSE 4552, JCR) today announced that the European Commission (EC) has granted orphan drug designation (ODD) to JR-446, an investigational drug for the treatment of mucopolysaccharidosis type IIIB (MPS IIIB or Sanfilippo syndrome type B), following the recent the U.S. Food and Drug Administration (FDA) designation.1
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · June 24, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, announced the launch of a film featured in The Next Frontier, a global branded film series presented by the Biotechnology Innovation Organization (BIO) and produced by BBC StoryWorks Commercial Productions. The Next Frontier series explores how biotechnology can be used effectively to address urgent challenges the world faces.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · June 18, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”), a global specialty biopharmaceutical company dedicated to developing therapies for rare and genetic diseases, today announced the launch of its new global website. As the company turns 50, it is marking its growing footprint in Japan, the U.S., Europe, and Latin America with a unique website tailored for global audiences that reflects its passion for both science and humanity and brings the JCR brand to life for patients, physicians, and partners worldwide.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · June 6, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) announced today that the Company presented preclinical data from its novel adeno-associated virus (AAV) gene therapy research programs at the American Society of Gene and Cell Therapy (ASGCT) 28th Annual Meeting, being held May 13-17, 2025, in New Orleans, LA. In an oral presentation, the JCR researcher reported that the Company’s proprietary J-Brain Cargo® (JBC) technology enables the efficient delivery of an adeno-associated virus (AAV) gene therapy across the blood-brain barrier (BBB) and into the central nervous system (CNS) in mice, monkeys, and several animal models of CNS diseases.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · May 16, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) announced today that it will present updated preclinical data from its proprietary J-Brain Cargo®-applied adeno-associated virus (AAV) vector gene therapy non-clinical research programs in an oral abstract session at the American Society of Gene and Cell Therapy (ASGCT) 28th Annual Meeting, being held May 13-17, 2025, in New Orleans, LA.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · May 8, 2025
MEDIPAL HOLDINGS CORPORATION (TSE 7459, MEDIPAL) and JCR Pharmaceuticals Co., Ltd. (TSE 4552, JCR) today announced that the U.S. Food and Drug Administration (FDA) granted orphan drug designation (ODD) to JR-446, an investigational drug for the treatment of mucopolysaccharidosis type IIIB (MPS IIIB or Sanfilippo syndrome type B).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · May 7, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) today announced the presentation of two datasets demonstrating the potential benefits of its investigational therapies for lysosomal storage disorders (LSDs) at the 21st Annual WORLDSymposiumTM 2025. JCR is presenting new data from a pair of its programs that apply its J-Brain Cargo® platform, a proprietary technology developed by JCR, to deliver medicines across the blood-brain barrier (BBB) through poster presentations this week.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 5, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) announced today that it will present at the 21st Annual WORLDSymposium™ 2025, held February 3-7, 2025, in San Diego, Calif. The poster presentations will demonstrate the potential benefits of the investigational therapies in JCR’s development pipeline and of J-Brain Cargo®, JCR’s proprietary technology that delivers medicine across the blood-brain barrier (BBB) for the treatment of lysosomal storage disorders and neurodegenerative disorders.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · January 28, 2025

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) today announced that the first patient has been dosed in the Phase III clinical trial of JR-142 (INN: redalsomatropin alfa) in Japan, marking a significant milestone in the development of this innovative treatment. JR-142 is a long-acting growth hormone therapy and is being studied in patients with pediatric growth hormone deficiency.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · December 20, 2024

MEDIPAL HOLDINGS CORPORATION (TSE 7459, MEDIPAL) and JCR Pharmaceuticals Co., Ltd. (TSE 4552, JCR) today announced the initiation of the Phase I/II clinical trial of JR-446 in Japan following the dosing of the first individual from the trial. JR-446 is a proprietary blood-brain barrier (BBB)-penetrating α-N-acetylglucosaminidase in development for the treatment of mucopolysaccharidosis type IIIB (Sanfilippo syndrome type B or MPS IIIB).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · December 5, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) announced the initiation of the first patient dosing in Japan in the Phase I clinical trial of JR-441, an investigational enzyme replacement therapy for the treatment of mucopolysaccharidosis type IIIA (MPS IIIA, also known as Sanfilippo syndrome type A). JR-441 is a proprietary recombinant heparan N-sulfatase capable of crossing the blood-brain barrier (BBB).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · October 31, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today that the Company presented preclinical data from its novel adeno-associated virus (AAV) gene therapy research programs in a poster session at the European Society of Gene and Cell Therapy (ESGCT) 31st Annual Congress, being held October 22-25, 2024, in Rome, Italy. JCR leverages its proprietary J-Brain Cargo® (JBC) technology for the delivery of adeno-associated virus (AAV) gene therapy to the body and the brain to potentially address the central nervous system (CNS) symptoms by crossing the blood-brain barrier (BBB).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · October 23, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today that it will present preclinical data from its proprietary J-Brain Cargo®-applied adeno-associated virus (AAV) gene therapy preclinical research programs in a poster session at the European Society of Gene and Cell Therapy (ESGCT) 31st Annual Congress, being held October 22-25, 2024, in Rome, Italy.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · October 16, 2024

JCR Pharmaceuticals Co., Ltd. (TSE: 4552) made significant contributions at the Society for the Study of Inborn Errors of Metabolism (SSIEM) Annual Symposium 2024, held in Porto, Portugal from September 3-6, 2024. We highlighted our proprietary J-Brain Cargo® technology through two key presentations focused on advanced therapies for lysosomal storage disorders, demonstrating our pioneering role in medical innovation.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 5, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today the completion of the regulatory review by the Pharmaceuticals and Medical Devices Agency (PMDA) for the clinical trial notification for the Phase I study of JR-441 in individuals with mucopolysaccharidosis type IIIA (MPS IIIA; Sanfilippo syndrome type A).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 3, 2024
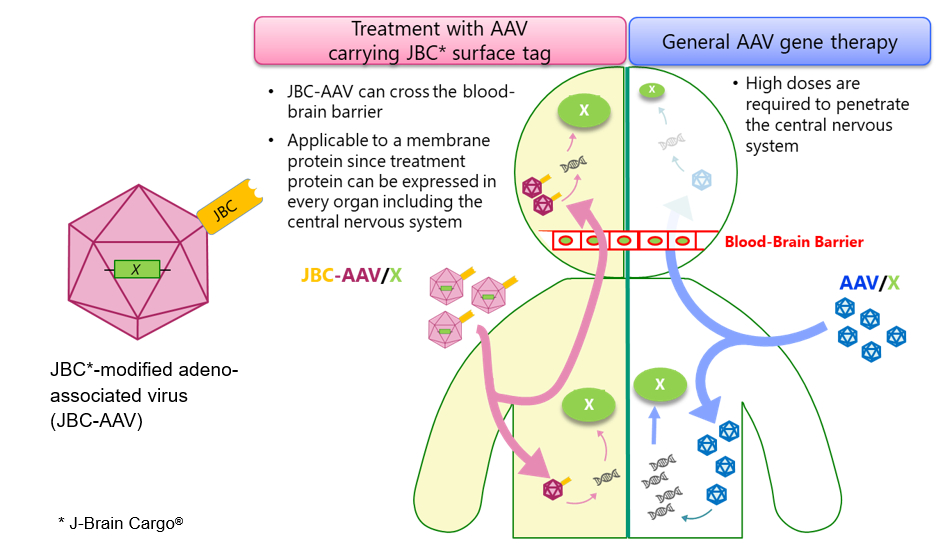
JCR Pharmaceuticals Co., Ltd. (TSE 4552: Chairman and President Shin Ashida, “JCR”) announced presentations highlighting its latest advancements in gene therapy research at the 7th International Forum of Lysosomal Disorders, held in Tokyo on July 12-13, 2024.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · July 18, 2024

MEDIPAL HOLDINGS CORPORATION (TSE 7459 “MEDIPAL”) and JCR Pharmaceuticals Co., Ltd. (TSE 4552 “JCR”) today announced the completion of the regulatory review by the Pharmaceuticals and Medical Devices Agency (PMDA) for the clinical trial notification for the phase I/II study of JR-446, a blood-brain barrier-penetrating α-N-acetylglucosaminidase, for the treatment of mucopolysaccharidosis type IIIB (MPS IIIB; Sanfilippo syndrome type B), a devastating and ultra-rare lysosomal storage disorder.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · July 2, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) today announced that it has achieved the first research milestone criterion in a collaboration with Alexion, AstraZeneca Rare Disease (“Alexion”) to develop an undisclosed initial therapeutic molecule that applies JCR’s proprietary J-Brain Cargo®, blood-brain barrier penetration technology, for the treatment of a neurodegenerative disease. (Related release is here). The achievement of a predefined research milestone triggers a milestone payment to JCR.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · March 19, 2024

Thursday, February 29 is Rare Disease Day (“RDD”). RDD activities aim to raise awareness of rare diseases and to improve the quality of life of individuals with rare and intractable diseases through better diagnoses and treatments. JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) endorses the important mission of RDD and will advocate for RDD 2024 in Japan.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 29, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) today announced the presentation of several datasets demonstrating the potential benefits of its investigational therapies for lysosomal storage disorders (LSDs). In a series of oral and poster presentations at the 20th Annual WORLDSymposium™ in San Diego, Calif., JCR highlighted several programs that rely on J-Brain Cargo®, a proprietary technology developed by JCR, to deliver medicines across the blood-brain barrier (BBB).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 14, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today that it will present six presentations at the 20th Annual WORLDSymposium™ 2024, to be held February 4-9, 2024 in San Diego, Calif. These presentations demonstrate the potential benefits of the investigational therapies in JCR’s development pipeline and of J-Brain Cargo®, JCR’s proprietary technology that delivers medicine across the blood-brain barrier (BBB) for the treatment of lysosomal storage disorders and neurodegenerative disorders.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · January 25, 2024

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today that Takeda Pharmaceutical Co., Ltd. (“Takeda”) decided to discontinue their collaboration with JCR to develop gene therapies using adeno-associated viruses (AAV) combined with the JCR J-Brain Cargo® Technology.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · December 21, 2023

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida: “JCR”) announces that it has signed a Research Collaboration, Option and License Agreement with Alexion, AstraZeneca Rare Disease (“Alexion”) for the development of novel oligonucleotide therapeutics to support targeted delivery to certain tissues or organs using J-Brain Cargo®, JCR’s proprietary blood-brain barrier (BBB)-penetrating technology.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · December 21, 2023

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced that the U.S. Food and Drug Administration (“FDA”) granted orphan drug designation (ODD) to JR-441, an investigational drug for the treatment of mucopolysaccharidosis type IIIA (MPS IIIA, or Sanfilippo syndrome type A). JR-441 is a blood-brain barrier (BBB)-penetrating form of recombinant heparan N-sulfatase that was developed using JCR’s proprietary J-Brain Cargo® BBB-penetrating technology.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · December 15, 2023

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today that the first patient in the Phase I/II clinical trial with Mucopolysaccharidosis Type IIIA (MPS IIIA; Sanfilippo Syndrome Type A) has been dosed with JR‑441, a blood-brain barrier (BBB) penetrating form of heparan N-sulfatase that was developed using JCR’s proprietary J-Brain Cargo® BBB-penetrating technology. JR-441 is a recombinant fusion protein of antibody fragment against the human transferrin receptor and heparan N- sulfatase, the enzyme missing or malfunctioning in subjects with MPS IIIA.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · November 10, 2023

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today key results from the 52-week interim data of its global phase I/II study with JR-171 (INN: lepunafusp alfa) in individuals with mucopolysaccharidosis type I (MPS I, also known as Hurler, Hurler-Scheie and Scheie syndrome). JR-171 is a blood brain-barrier (“BBB”)-penetrating form of recombinant α-L-iduronidase that was developed using JCR’s proprietary J-Brain Cargo® technology. There are no approved therapies that cross the BBB and address the central nervous system (CNS) symptoms for individuals with MPS I.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · September 29, 2023

Angelini Pharma, part of the privately owned Angelini Industries, and JCR Pharmaceuticals Co., Ltd. (TSE 4552; “JCR”) announced today that they entered into an exclusive global development and commercialization agreement for the development of novel biologic therapies that applies J-Brain Cargo®, blood-brain barrier penetrating technology, for the treatment of epilepsy.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · May 11, 2023

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) today announced that JCR has signed a Research Collaboration, Option and License Agreement with Alexion, AstraZeneca Rare Disease (“Alexion”) to develop an undisclosed initial therapeutic molecule that applies JCR’s proprietary J-Brain Cargo®, blood-brain barrier (“BBB”) penetration technology, for the treatment of a neurodegenerative disease.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · April 3, 2023

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) today announced the presentation of several datasets demonstrating the potential benefits of its investigational therapies for lysosomal storage disorders (LSDs). In a series of oral and poster presentations at the 18th Annual WORLDSymposium™ in San Diego, Calif., JCR highlighted the potential benefits of therapies that rely on J-Brain Cargo®, a proprietary technology developed by JCR Pharmaceuticals, to deliver medicines across the blood-brain barrier (BBB).
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 15, 2022

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) announced today that it will present six posters at the 18th Annual WORLDSymposiumTM 2022, to be held February 7-11, 2022 in San Diego, Calif. These presentations demonstrate the potential benefits of the investigational therapies in JCR’s development pipeline – and of J-Brain Cargo®, JCR’s proprietary technology that delivers medicine across the blood-brain barrier (BBB) – for the treatment of lysosomal storage disorders.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 3, 2022

JCR Pharmaceuticals Co., Ltd. (TSE 4552; Chairman and President: Shin Ashida; “JCR”) today announced that WORLDSymposium™ 2022 has bestowed its New Treatment Award to IZCARGO® (pabinafusp alfa 10 mL, intravenous infusion), which the Ministry of Health, Labour and Welfare (MHLW) approved last year for the treatment of mucopolysaccharidosis type II (MPS II, or Hunter syndrome) in Japan. IZCARGO® (formerly known as JR-141) is a recombinant iduronate-2-sulfatase enzyme replacement therapy (ERT) that relies on J-Brain Cargo®, a proprietary technology developed by JCR, to deliver therapeutics across the blood-brain barrier (BBB). It is the first-ever approved ERT in any country that penetrates the BBB via intravenous administration, a potentially life-changing benefit for individuals with lysosomal storage disorders (LSDs) such as MPS II.
By JCR Pharmaceuticals Co., Ltd. · Via Business Wire · February 3, 2022
